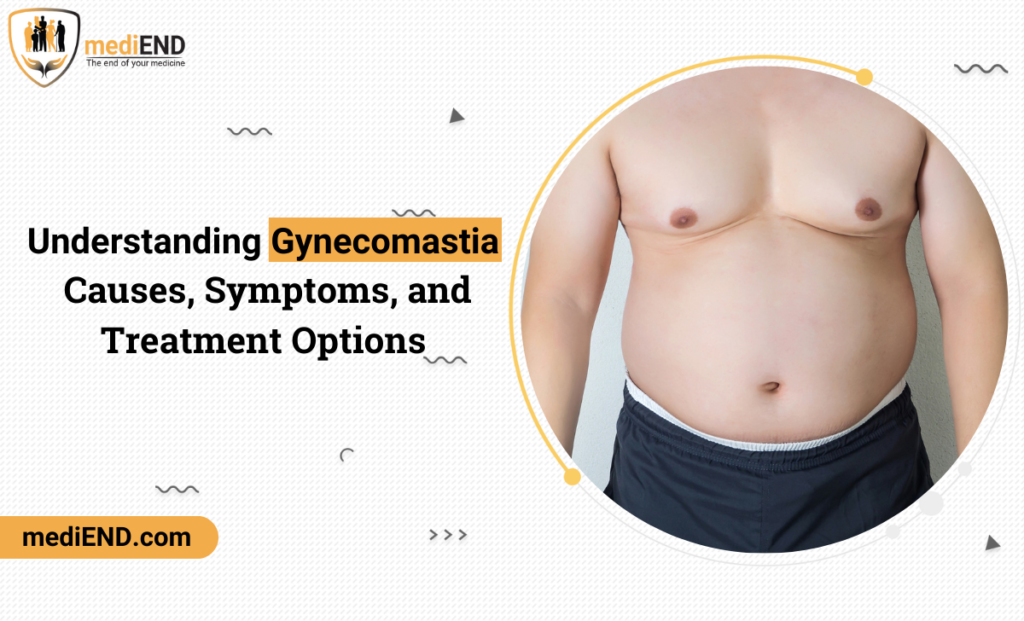
These aggregate safety reports include the cumulative summary of safety information for molecules under clinical development. They usually include safety information from nonclinical studies and safety data of the subjects who participated in the clinical trial services.
Regulatory Consulting Services
Medical device consulting Services