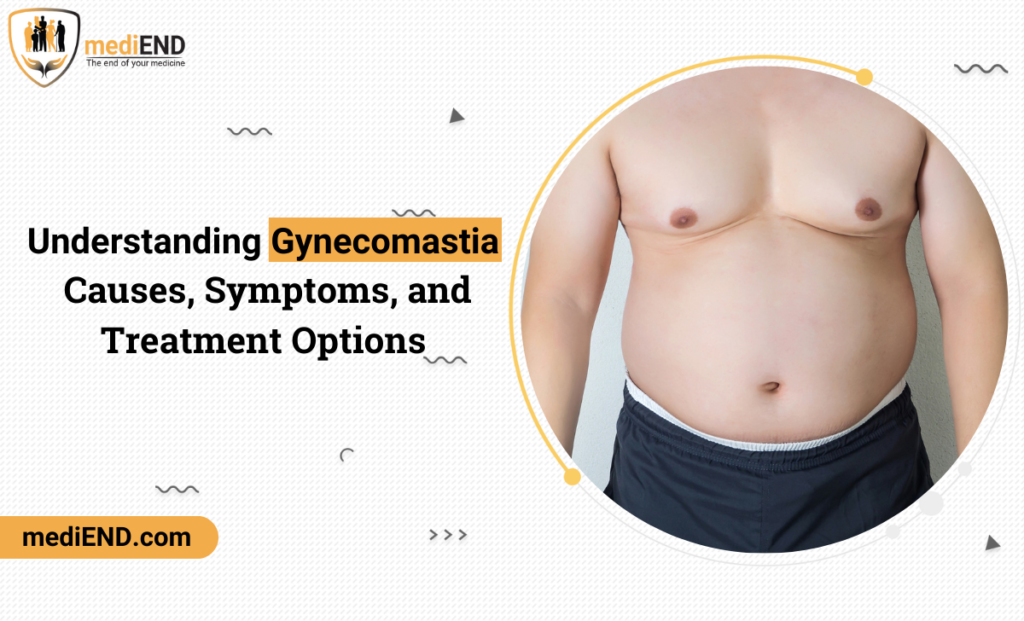
A CDx assay, according to the US-America Food and Drug Administration (FDA) guidance, is defined as an IVD device that provides information that is essential for the safe and effective use of a corresponding therapeutic product. It is required when drug/biologic has a specific genetic or biological target not present in all patients with a particular disease.
Check more details: Companion Diagnostics (CDx) Development